SEARCH
CONTACT US
- Office Add: No.18 Wenchang West Rd, Yangzhou City, 225009, Jiangsu Province, P.R.China
- Tel: 0086-514-82235318
- Fax: 0086-514-87875333
- E-mail: admin@sinoguotai.com
- Msn: sinoguotai@foxmail.com
Products Info
Product Description
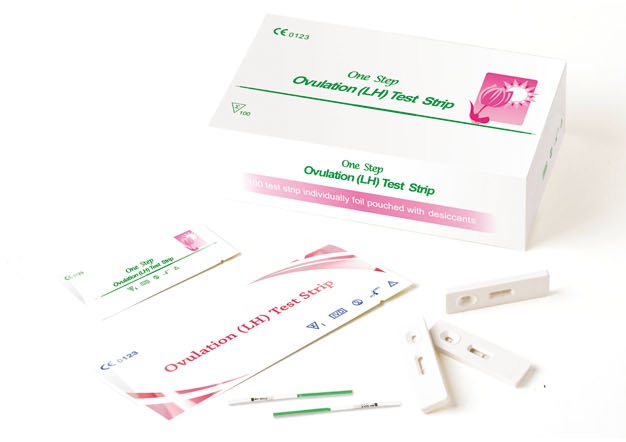
LH Test Kits include strips, cassettes and midstreams.
Human luteinizing hormone (hLH) is a glycoprotein hormone secreted by the anterior pituitary. HLH, hFSH together with other steroid hormones are known to play important roles in regulating ovulation and other ovarian functions during the menstrual cycle. Maturation of an ovarian follicle and its oocyte begins during the end of the preceding menstrual cycle. In response to hFSH released by the pituitary, the follicle undergoes rapid growth. As follicles develop, estradiol secretion begins to rise slowly and is followed by a rapid increase. This increase of estradiol level is generally believed as the trigger for the rapid rise and peaking of hLH activity at the mid-cycle (hLH surge). Approximately 12-24 hours after the hLH surge, the wall of the enlarged follicle ruptures at ovulation and the mature ovum is extruded. After ovulation, hLH returns to its base line level within two days with the concomitant increase of progesterone level to initiate luteal phase. The luteal phase lasts predictably about 14 days. Unless pregnancy occurs, a new follicle begins the selection procedure for maturation in the next menstrual cycle.
In view of the characteristic variation of hLH during the menstrual cycle, rapid and sensitive measurement of hLH is an important tool in the diagnosis and management of infertility in females. Detection of the hLH surge can aid in predicting the time of ovulation. The onset of the hLH surge proceeds ovulation by approx. 30 hours. The analysis of hLH has been used successfully to time oocyte retrieval for in vitro fertilization and would similarly assist timing of artificial insemination.
1. Read directions for use carefully before performing this test. Pay attention to the position of the C and T line.
2. Do not use kit beyond the expiration date.
3. Samples may be infectious; properly handle and dispose of all used reaction devices in the biohazard container.
4. For in vitro diagnostic use only.